FDA: Pharmacy's other drugs may be causing illness
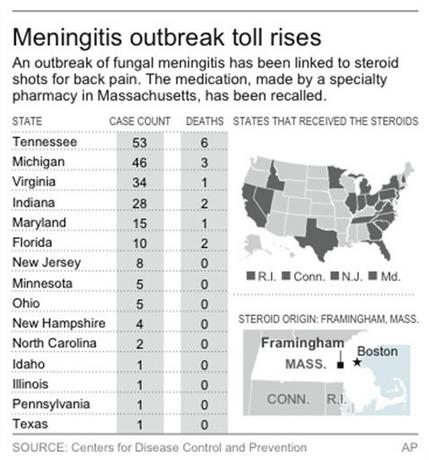

Map shows states affected by the meningitis outbreak and those receiving suspected tainted medications.
October 15. Two more drugs from a specialty pharmacy linked to a meningitis outbreak are now being investigated, U.S. health officials said, as they urged doctors to contact patients who got any kind of injection from the company.
The New England Compounding Center of Framingham, Mass., has been under scrutiny since last month, when a rare fungal form of meningitis was linked to its steroid shots used mostly for back pain.
Monday’s step by the Food and Drug Administration followed reports of infections in three people who got different drugs made by the company. One is a possible meningitis illness in a patient who got a spine injection of another type of steroid. The agency also learned of two heart transplant patients who got fungal infections after being given a third company product during surgery.
The illnesses are under investigation, and it’s very possible the heart patients were infected by another source, FDA officials cautioned. They did not say whether the meningitis case involved a fungal infection or where the three patients lived.
As of Monday, the current outbreak has sickened 214 people, including 15 who have died, in 15 states. For weeks, officials have been urging doctors to contact patients who got shots of the company’s steroid methylprednisolone acetate, advise them about the risks of fungal infection, and urge them to take any meningitis symptoms seriously.
The steroid was recalled last month, and the company later shut down operations and recalled all the medicines it makes.
The FDA on Monday expanded its advice to doctors to contact all patients who got any injection made by the company, including steroids and drugs used in eye surgery as well as heart operations. The agency said it took the step “out of an abundance of caution” as it investigates the new reports involving the heart surgery drug and the second steroid, called triamcinolone acetonide.
The company issued a statement Monday that said it was reviewing the FDA’s latest advisory, but is continuing to cooperate with the FDA and other federal and state agencies looking into the outbreak.
“As we have said, we will respect those public agencies’ processes for investigations and will not comment while they are under way,” the statement said.
Nearly all the 214 illnesses in the outbreak are fungal meningitis; two people had joint infections.
Last week, federal health officials said 12,000 of the roughly 14,000 people who received the steroid shots had been contacted. Those people received methylprednisolone acetate injections at clinics in 23 states.
New England Compounding, which custom-mixes ointments, painkillers and other products, is licensed to sell in all 50 states. The FDA did not say how many patients fall under the new advisory, or where the products were shipped.
Symptoms of meningitis include severe headache, nausea, dizziness and fever. The CDC said many of the cases have been mild, and some people had strokes. Symptoms have been appearing between one and four weeks after patients got the shots, but CDC officials on Thursday warned at least one illness occurred 42 days after a shot.